Increased Deaths and Eye Removals Result from Recalled Eye Drops
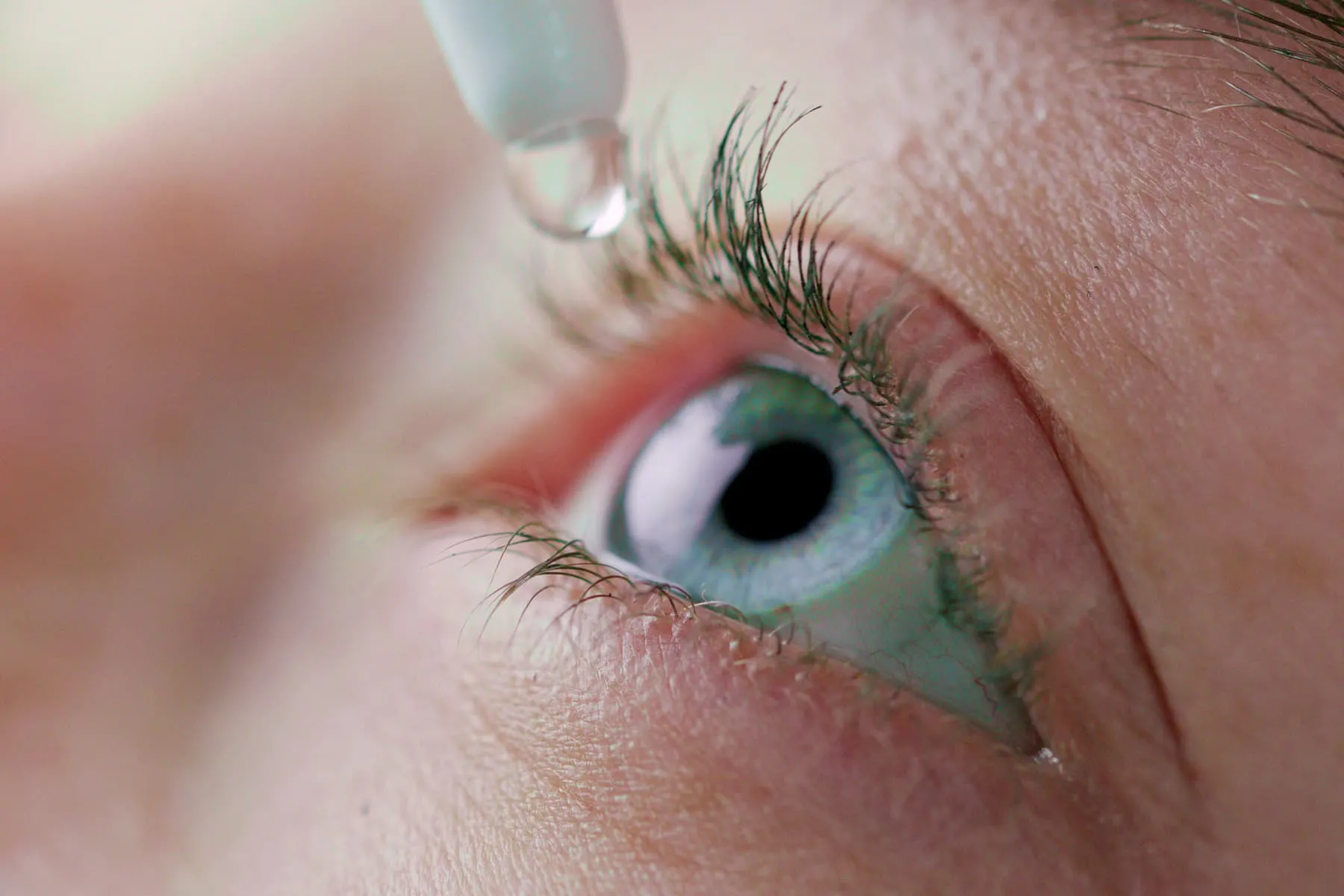
On March 23rd, 2023, additional individuals passed away due to using eye drops that were tainted with a type of bacteria that is highly resistant to numerous types of drugs. The CDC has referred to this bacteria as an unusual strain.
Earlier this year, the eye drops and ointments offered by EzriCare and Delsam Pharma were withdrawn or taken back from the market.
As of now, three individuals have passed away, while eight individuals have experienced vision impairment and four people had to undergo a surgical procedure for eye removal known as enucleation. The situation has worsened over the past month as initially, only one death was reported due to the outbreak.
According to the latest update from the CDC, a total of 68 individuals, spread across 16 different states, have been impacted by the contaminated eye drops. The cases took place between May 2022 and February 2023. The majority of those affected, exceeding half of the cases, are reported to be connected to four separate incidents that happened in health care centers. A spokesperson for the CDC mentioned that most of these individuals had already displayed symptoms by the time the center issued its official warning on Jan. 20.
Tests conducted in the laboratory have established that the bacterium P. aeruginosa was found to be present in EzriCare products bottles of both infected and non-infected patients.
The CDC declared that they are presently carrying out examinations on EzriCare Artificial Tears bottles that have not been opened to help them decide if there has been any contamination during production.
The products that were taken off the market include EzriCare's man-made eye solution, as well as Delsam Pharma's man-made eye solution and eye ointment. The Centers for Disease Control and Prevention (CDC) stated that the majority of people affected had used man-made eye solutions, with the most frequent one being "EzriCare Artificial Tears", which can be bought without a prescription and comes in bottles for multiple uses that do not contain preservatives. This same product was also used during four separate incidents at healthcare facilities, which resulted in a total of 37 cases.
If you're using the products and notice any of the symptoms of an eye infection, make sure to seek medical attention as soon as possible.
Currently, the CDC does not have any guidelines for individuals who did not experience any symptoms of eye infection after using these products.

























































