CDC Alert: Drug-resistant bacteria in eyedrops result in 1 death and others forced to have eyes removed.
Stay vigilant about this unsettling unfolding tale.
According to the Centers for Disease Control and Prevention, a total of 68 individuals across 16 states in the United States have received a diagnosis of a rare bacterial infection that is believed to have been caused by the usage of eyedrops that do not contain preservatives.
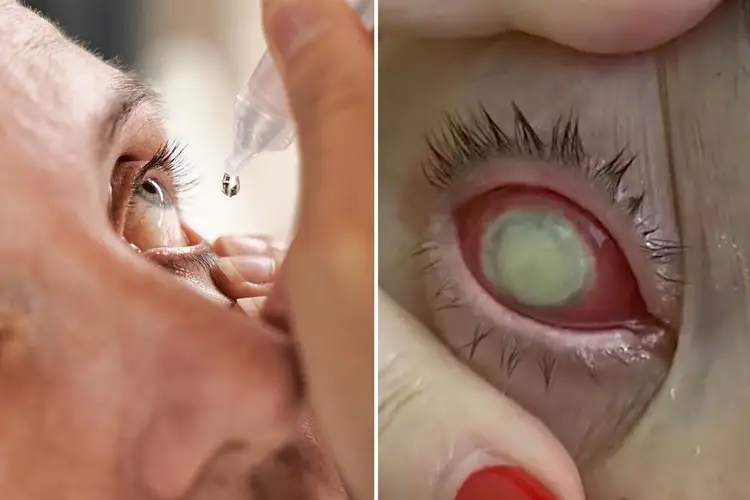
The agency responsible for monitoring public health has reported that a type of Pseudomonas aeruginosa that is not commonly seen and cannot be treated with antibiotics has caused the passing of one individual in the United States. Additionally, this strain of Pseudomonas aeruginosa has caused eight other patients to lose their sight.
EzriCare Artificial Tears manufacturers are facing a lawsuit from Clara Oliva, a grandmother from Florida, who was forced to undergo surgical removal of her eyes due to infection along with three other individuals.
Natasha Cortes, the attorney for Oliva, stated via email to Law&Crime that her client has sustained severe injuries and is now legally blind. She is actively examining other cases where individuals have been similarly injured by the product that has since been recalled.
It is important to ensure that these companies are made responsible for the terrible outcomes that their products have led to for Ms. Oliva and other people who bought them.
In the early part of the year, the CDC advised individuals to discontinue the use of a certain product, as well as Delsam Pharma’s Artificial Tears and Ointment, due to the discovery of harmful bacteria in opened bottles collected from clients.
According to Cortes' statement to NBC, the individual from Florida regularly employed EzriCare Artificial Tears prior to contracting the infection.
During an interview on Tuesday, the legal expert informed the news network that the product doesn't include preservatives, which are typically utilized to combat bacterial infections.
There are probably a large number of individuals who have experienced infections without realizing it, just as Ms. Oliva did.
Based on the legal claim filed by Oliva, a grandmother aged 68, she commenced using EzriCare Artificial Tears in May of the preceding year.
After a number of months, she noticed that her right eye had become inflamed and excessively watery. Eventually, she contracted a bacterial infection that resulted in a corneal ulcer and a decline in her ability to see clearly.
According to the legal complaint, due to the seriousness of the infection in Mrs. Oliva's right eye, the failure of various treatment methods, and the possibility of the infection spreading throughout her body and becoming life-threatening, the decision was made to remove her right eye through enucleation in order to manage the extremely resistant infection.
Mrs. Oliva underwent an operation on September 1, 2022 to remove her right eye and insert a plastic replacement. As a result, her vision has significantly decreased, with 20/200 visual acuity in her remaining left eye, leading to her classification as legally blind.
At this time, the CDC has not disclosed any confidential details regarding the individual who passed away after being infected with the uncommon variation of Pseudomonas aeruginosa.
Back in January, the company issued a caution to its clients to discontinue the usage of EzriCare Artificial Tears and Delsam Pharma's Artificial Tears as they had discovered an uncommon occurrence of infections.
Global Pharma Healthcare, the manufacturer of those two products, decided to issue a voluntary recall in February after receiving a recommendation from the Food and Drug Administration.
Nevertheless, a representative from EzriCare Artificial Tears expressed that despite conducting tests, they have yet to establish a conclusive correlation between their products and the spread of Pseudomonas aeruginosa.
The representative of the company said that they have been reaching out to their customers as much as they can to discourage them from using the product any further.
We also promptly contacted the CDC and FDA and expressed our readiness to collaborate with any demands they may have for us.





























































