AstraZeneca: What are the rare side effects of the Covid jab?
Get Exclusive Health Analysis In Our Free Email Check
Free Health Check By Email
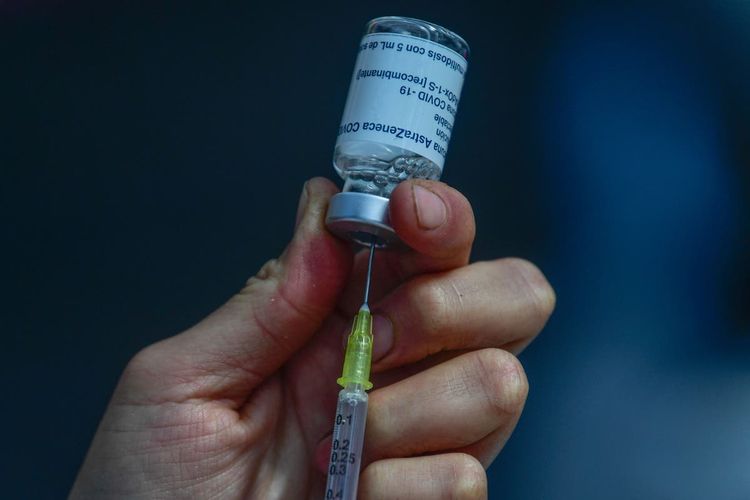
AstraZeneca has declared that it has commenced a cancellation of its Covid-19 vaccine across the globe, after acknowledging several months ago that the medication could result in uncommon yet fatal harm.
The vaccination, once referred to as Covishield, was created through a partnership between an enormous pharmaceutical company and Oxford University. It was manufactured by Serum Institute of India.
The company from Britain and Sweden announced that they plan to take away the approval for selling the vaccine called Vaxzevria in Europe.
The European Medicines Agency (EMA) declared on Tuesday that the vaccine is not approved for utilization anymore.
The Covid vaccine has been given out in more than 150 nations, which includes the United Kingdom and various parts of the European Union.
The vaccine has been withdrawn due to there being too many updated vaccines available since the pandemic began, according to the company.
Several research studies carried out during the pandemic have discovered that the vaccination is capable of providing between 60 and 80 percent effectiveness in safeguarding against Covid.
What are the repercussions of getting the vaccine?
Some new studies have discovered that Covishield can lead to the formation of blood clots in certain individuals. These clots could potentially be life-threatening.
In February, AstraZeneca acknowledged in legal papers presented to the High Court that their vaccine might lead to a condition called Thrombosis with Thrombocytopenia Syndrome (TTS) in extremely exceptional circumstances, as stated by The Telegraph. Nevertheless, the origin of this ailment is still not evident.
Additionally, text-to-speech syndrome can also happen without the involvement of the AZ vaccine or any other vaccination. Determining the cause for each specific case will rely on the analysis of an expert.
TTS is identified by the occurrence of blood clots and a reduced amount of blood platelets in the human body.
The statement asserted that the vaccine caused fatalities and serious harm and requested compensation of up to £100m for approximately 50 affected individuals.
One of the people who filed a complaint claimed that the vaccine permanently harmed their brain, which happened when they had a blood clot and made it impossible for them to work.
It has been reported that a unique syndrome has been observed in approximately two to three individuals out of every 100,000 who received the Vaxzevria vaccination.
The vaccine has been confirmed by the World Health Organization to potentially cause deadly aftermaths.
The World Health Organization (WHO) stated that a really uncommon undesirable occurrence named Thrombosis with Thrombocytopenia Syndrome has been discovered in relation to the vaccine. This syndrome triggers exceptional and severe blood clots while also causing a decrease in platelet numbers.
The Council for International Organizations of Medical Sciences defines "very rare" side effects as those that are reported in less than 1 case out of 10,000.
The World Health Organization stated that in places where SARS-CoV-2 is still spreading, getting vaccinated provides much greater advantages in safeguarding against Covid-19 than any potential drawbacks.
How common was the administration of the vaccine in the UK?
By the autumn of 2021, approximately 50 million injections of the Oxford-AstraZeneca vaccine had been given in the United Kingdom.
Although the government had previously administered the jab, it was predominantly discontinued and subsequently replaced by the Pfizer and Moderna vaccines during the winter booster campaign towards the end of 2021.
The drug manufacturer stated that they are not pulling the vaccine due to the legal proceedings.
AstraZeneca has made a statement expressing their great pride in the contribution that Vaxzevria has made in putting an end to the global pandemic. They have stated that through independent evaluations, more than 6.5 million lives were saved solely in the first year of usage, and over three billion doses were provided worldwide.
Governments all over the planet have acknowledged our endeavors and consider them as a vital element to put an end to the worldwide epidemic.
Due to the emergence of several Covid-19 vaccines, there are now more updated options for people to choose from. As a result, there is less interest in the Vaxzervria vaccine, causing its production and distribution to cease. AstraZeneca has decided to proceed with the removal of Vaxzevria's Marketing Authorisation in Europe.
We will collaborate with regulators and our associates to establish a definite course of action to bring this episode to a close and make a noteworthy contribution to the fight against Covid-19 outbreak.































































