AstraZeneca: What are the rare side effects of the Covid jab?
Get A Free Health Check Email For Weekly Health Insights
"Free Health Check Email: Sign Up Now"
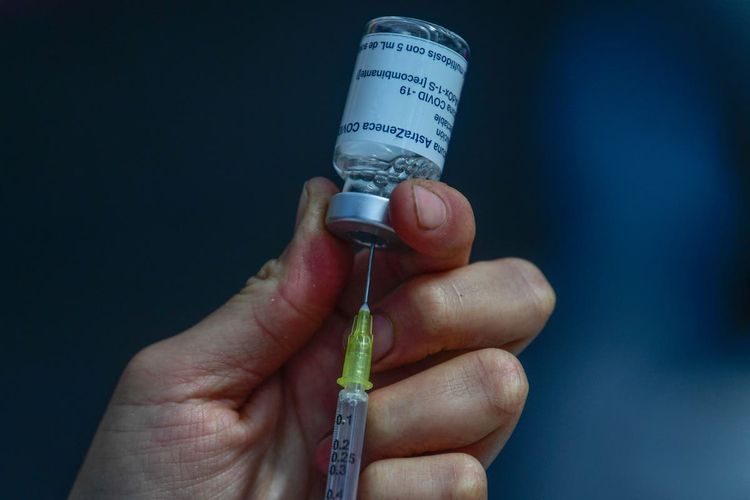
A global recall of its Covid-19 vaccine has been initiated by AstraZeneca, after acknowledging a few months before that the medicine has the potential to produce life-threatening injuries, though they were rare.
The vaccination, previously referred to as Covishield, was formed by the pharmaceutical corporation along with Oxford University, and made by the Serum Institute of India.
The company from the UK and Sweden has stated that they will continue to remove the authorization to market their vaccine, Vaxzevria, in Europe.
The European Medicines Agency issued a statement on Tuesday that the vaccine is not permitted to be used anymore.
The Covid vaccine was extensively distributed across more than 150 nations, encompassing the UK and throughout the European Union.
The reason for the vaccine being withdrawn is due to an excess of more recent and readily available vaccines resulting from the pandemic, according to the company's statement.
Several researches carried out during the pandemic discovered that the vaccine had a success rate of 60 to 80 percent in shielding individuals from Covid.
What are the adverse reactions caused by the vaccine?
New studies reveal that Covishield has the potential to trigger blood coagulation in certain individuals, leading to life-threatening consequences.
AstraZeneca has stated in legal papers submitted to the High Court in February that their vaccine can cause Thrombosis with Thrombocytopenia Syndrome (TTS), but this only occurs in extremely rare cases. The exact reason for this side effect is still unclear. This information was reported by The Telegraph.
Moreover, TTS may occur even without the AZ vaccine or any other vaccine. The establishment of causality in each specific case will require the testimony of an expert.
The medical condition known as TTS is identified by the presence of blood clots and a deficiency in the number of platelets within the bloodstream of individuals.
The objection alleged that the vaccine caused fatalities and grave harm and requested compensation of up to 100 million pounds for roughly 50 individuals who were affected.
One of the people who filed a complaint claimed that the vaccine gave them a lasting brain injury by causing a blood clot, which has stopped them from being able to work.
The uncommon condition was noticed in roughly 2-3 individuals out of 100,000 who received the Vaxzevria immunization.
The vaccine was verified by the World Health Organization to have potential fatal consequences.
According to the World Health Organization (WHO), vaccination with this specific vaccine has caused an infrequent and serious occurrence known as Thrombosis with Thrombocytopenia Syndrome. This issue is linked to rare and severe clotting episodes and abnormally low levels of platelets in the blood.
As stated by the Council for International Organizations of Medical Sciences, side effects that are deemed as "very rare" only appear in fewer than 1 out of 10,000 instances.
The World Health Organization stated that in nations where SARS-CoV-2 is still spreading, getting vaccinated is much more advantageous in safeguarding oneself from Covid-19 than the potential dangers it poses.
What was the extent of the vaccine's usage in the United Kingdom?
Approximately 50 million shots of the Oxford-AstraZeneca vaccine were given to people in the United Kingdom by the fall of 2021.
Yet the administration considerably ceased administering the vaccine, and instead, Pfizer and Moderna vaccines were utilized in the United Kingdom for the winter booster program towards the conclusion of 2021.
The drug manufacturer stated that taking back the vaccine has no connection to the legal proceedings.
AstraZeneca announced that they are very pleased with how Vaxzevria has contributed to stopping the worldwide pandemic. Based on reports from independent sources, Vaxzevria has saved more than 6.5 million lives in the first year it was utilized, and over three billion doses have been given to people all over the world.
Governments worldwide have acknowledged and appreciated our endeavors, considering them as a pivotal element in bringing the global pandemic to a halt.
With the creation of different kinds of coronavirus vaccines, there are now more than enough updated ones to go around. Because of this, there aren't as many people wanting to use Vaxzevria anymore and it's not being made or given out like it used to be. AstraZeneca has decided to stop selling Vaxzevria within Europe and get rid of its permits to do so.
Currently, we're collaborating with regulators and our associates to establish a definite plan to wrap up this phase and make a substantial impact towards the fight against Covid-19.































































